Organ-on-chip
Existing organ-on-chip models
Kidney-on-a-chip
The kidney is one of the most complicated organs in the human body. It, therefore, presents an enormous challenge for researchers to develop a platform of “kidney-on- a-chip” that can mimic its function when exposed to various drugs and medications.</centre>
An on-chip nephron device made out of a single multilayered polymer construct has been proposed by Weinberg et al. The chip resembles the functioning nephron with three sections, viz. the glomerulus, the proximal tubule and the loop of Henle and is made up of two microfabricated layers separated by a membrane. The filtration of the glomerular unit, the reabsorption in the tubule, and the large increase in urea concentration in the loop of Henle were all observed when a blood sample was introduced into the on-chip nephron device. This was one of the first attempts in the field. Though it was a successful attempt, there were challenges to be addressed related to the culture and control of the various cell types and the filtration process for the separation of waste was not as efficient as for the normal nephron.
Human breathing-lung-on-a-chip
The lung-on-a-chip is a complex, three-dimensional model of a living, breathing human lung on a microchip. The device is made using human lung and blood vessel cells and it can predict absorption of airborne nanoparticles and mimic the inflammatory response triggered by microbial pathogens.
Currently two research groups have come up with lung-on-chip. The alveolus lung-chip emulates fundamental lung functions of the alveolus, such as gas exchange and absorption. It has been used in a range of applications, including evaluation of nanoparticle absorption and toxicity, study of disease development and assessment of adverse drug effects, such as pulmonary edema and pulmonary thrombosis. The airway lung-chip (AwLC) recapitulates the physiology and function of the airway epithelium, which conducts inhaled air to the alveolar air sacs.
Pancreas-on-a-chip
The design of the islet-on-a-chip was inspired by the human pancreas, in which islands of cells (“islets”) receive a continuous stream of information about glucose levels from the bloodstream and adjust their insulin production as needed. Before transplanting beta cells into a patient, they must be tested to see whether they are functioning properly. The current method for doing this is based on technology from the 1970s: giving the cells glucose to elicit an insulin response, collecting samples, adding reagents and taking measurements to see how much insulin is present in each one. The manual process being highly time consuming to run and interpret may cause clinicians to not pursue it. The new, automated, miniature device gives results in real time enables speeding up clinical decision making.
Foetal membranes-on-a-chip
Preterm birth (PTB) is a leading cause of premature death in new-borns and children under the age of five. While the causes and the process triggering PTB are still not understood, 70% of the PTB cases have been correlated with bacterial infection of the foetal membrane, so called “chorioamnionitis” (CAM). CAM does not lead to premature rupture of the foetal membranes (PPROM) and this is possibly due to the innate maternal and foetal response. In order to clarify the origin of PPROM and the spatio-temporal progress of CAM a new human model of the foetal membranes are developed where bacterial infection and propagation through the foetal membrane can be observed and studied.
Proposed multi Organ-on-Chip
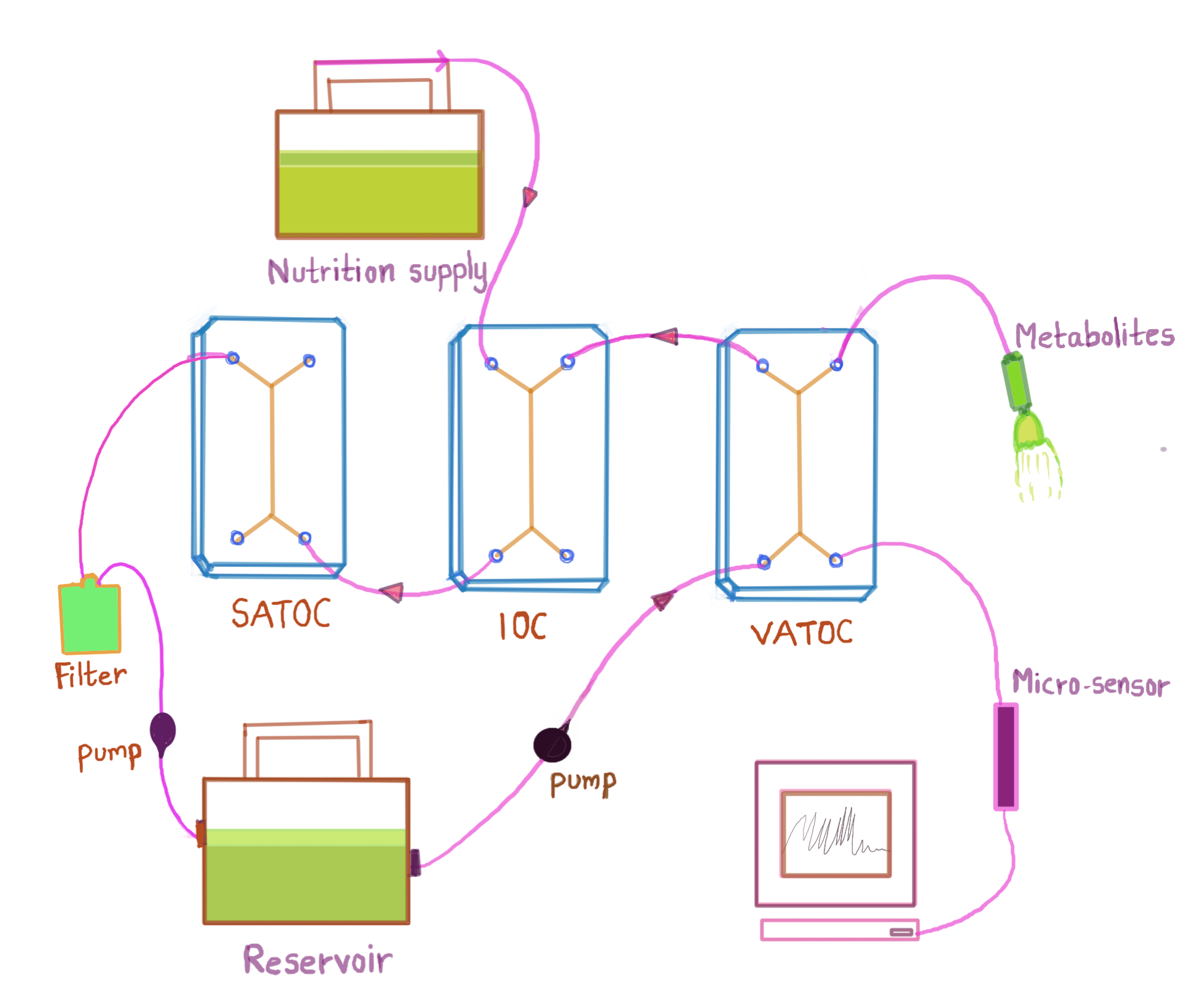
The design of the MOOC is proposed to mimic the structure and function of adipocytes and macrophages as adipose-tissue-on-chip (VATOC and SATOC) and β- cells and PDECs on IOC, as in FIG.

Each OOC comprises of two-cell culture chambers and a thin layer of porous membrane and are interconnected through the wells in each chip, allowing fluid flow between the chips. In ATOC, adipocytes (in VATOC, visceral adipocytes and in SATOC, subcutaneous adipocytes) are cultured in the top chamber and macrophages in the bottom chamber along with epithelial and endothelial cells as in FIG 5a. These macrophages are involved in obesity-associated islet inflammation and are responsible for various functions such as decreasing insulin secretion from β-cells and stimulating β-cell proliferation. This dominant immune cell type in IoL modulates β-cell function in obese individuals. In IOC, β-cells of the islets of pancreas, which are normally resident in the IoL are cultured with PDECs. The sampling can be done for the following, and modelling is performed: i) adipokines (leptin, adiponectin, adipsin), ii) pro-inflammatory factors (IL-6 and TNF-α), iii) anti-inflammatory factors (IL-1 receptor antagonist, IL-4, IL-10, IL-11, and IL-13), iv) insulin and v) glucose The metabolic activities described above will result in the generation of waste products. In the absence of an excretory system, it would be necessary to remove the waste products through filtration so that they do not impair the functioning of the OOCs.
MOOC may aid the study of mechanisms that regulate β-cell adaptation to obesity and the pathways me- diating the crosstalk between AT and β-cells. By administering a pulse of glucose into the top chamber of ATOC, inter-tissue effects of adipocytes on β-cell, direct effects of leptin, adiponectin and adipsin on the islet function, local effects of other adipokines can be studied and validated [13]. The change in key metabolites in time-series can be understood by the untargeted metabolomics approach. This can ultimately lead to a better understanding of the association of obesity-linked visceral and ectopic fat accumulation leading to the pathogenesis of diabetes [12]. As paracrine effects are sensitive it is not feasible to monitor and quantify the release of small concentration of substrates visually. With MOOC, this can be achieved to a great extent